What Does the Medical Oncologist Expect from the Pathologist in Order to Treat a Metastatic Non-Small Cell Lung Cancer?- Juniper Publishers
Juniper Publishers- Open Access Journal of Annals of Reviews & Research
What Does the Medical Oncologist Expect from the Pathologist in Order to Treat a Metastatic Non-Small Cell Lung Cancer?- Juniper Publishers
Authored by Georges El Hachem
Abstract
In the last decade, researchers provided us with significant advances in our understanding of lung cancer biology and management. They were able to identify many key driver events in lung carcinogenesis, resulting in the discovery of new modalities of targeted therapies towards a personalized medicine. In the same perspective, many clinical trials, together with the immunotherapy are including the patients according to the tumor mutational status and the expression of certain receptors or ligands. Thus, the pathological diagnosis is of high clinical relevance. Moreover, the pathologist is becoming an integrated and essential member during the multi-disciplinary team discussions, enriching our oncologic knowledge with a more developed and structured classification of non-small cell lung cancer. Here, in this review, we will discuss and list what Medical Oncologists are expecting to find in a pathology report in order to adequately treat the patients suffering from a metastatic non-small cell lung cancer.
Keywords: Non-small cell lung cancer; Mutation; Translocation; Targeted therapy
Abbrevations: FISH: Fluorescence In Situ Hybridization; IHC: Immune Histochemistry; RT-PCR: Reverse Transcriptase-Polymerase Chain Reaction; NGS: Next Generation Sequencing; TNM: Tumor Nodes Metastasis; SCLC: Small Cell Lung Cancer; EGFR: Epidermal Growth Factor Receptor; ALK: Anaplastic Lymphoma Kinase; TKIs: Tyrosine Kinase Inhibitors; TTF1: Thyroid Transcription Factor 1; DDR2: Discoidin Domain Receptor Tyrosine Kinase; NTRK1: Neurotrophic Receptor Tyrosine Kinase 1; MET: Mesenchymal Epithelial Transition; PDL1: Programmed Death Ligand 1; RET: Rearranged during Transfection
Introduction
Non-small cell lung cancers (NSCLCs) account for 85%-90% of lung cancers, while small cell lung cancers (SCLCs) are relatively decreasing in frequency in many countries over the past two decades [1]. The changes in the tumor-nodes-metastasis (TNM) classification headed towards a major change in the management of the NSCLC. Besides these prognostic modifications and the creation of the ‘’oligometastatic disease’’ entity, the current therapeutic decision relies on the following pathologic assessment: the morphologic description and the immunohistochemistry to define the histologic subtype, in addition to the molecular mutation/translocation identification.
The discovery of these mutations was practice changing in the metastatic setting only. It drastically changed the prognosis, not only improving the survival, but also the quality of life. However, in the early operable stage and other locally advanced stages which are treated with surgery and concomitant chemo-radiation therapy respectively, the pathologist is mainly responsible to identify the histological subgroup, and to exclude a small cell lung cancer. Controversially, it may be important to have the status of different molecular analyses (wild vs mutated) and other receptors expression that will serve as tumor archive. Upon disease recurrence, the Medical Oncologist will have a tumor specific platform to make an adequate therapeutic decision, especially if the metastatic site is not accessible to get a new specimen.
Discussion
NSCLCs are classified into adenocarcinoma, squamous cell lung carcinoma and adenosquamous lung carcinoma. Adenocarcinoma is the most common histological sub-type of lung cancer in contemporary series, accounting for approximately one-half of lung cancer cases [2]. Squamous-cell carcinoma comprises 25–30% of all lung cancer cases [3]. The reported incidence of adenosquamous carcinoma ranges from 0.4-4% of bronchogenic carcinomas [4].
1. Morphology and immunohistochemistry
Adenocarcinoma consists of four subtypes: acinar, papillary, adenocarcinoma in situ, and solid with mucous formation. They consist of glandular tumor cells producing mucous, and they grow in the peripheral part of the lung. They usually stain positive for thyroid transcription factor 1 (TTF1), cytokeratin 7 (CK7) and are negative for cytokeratin 20 (CK20) [5]. Controversially, squamous cell carcinoma consists of cells producing keratin in the same way as normal squamous epithelial cells. Histochemically, they tend to be TTF-1 negative, but positive for CK 5, CK 6 and P63 or P40. Through the tumor genesis, it was demonstrated that they develop from a pre-cancerous lesion as following: hyperplasia, metaplasia, dysplasia then carcinoma. These tumors generally grow in the central part of the lung in close relation to the large bronchi [6].
2. Targeted mutations and translocations
The identification of mutations in the epidermal growth factor receptor (EGFR) or rearrangements of the anaplastic lymphoma kinase (ALK) gene or proto-oncogene tyrosine-protein kinase ROS1 gene have led to a paradigm shift in the treatment of lung cancer by developing specific molecular therapies.
In NSCLC, the analysis of epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) inversions/ translocations are prerequisites for determining the appropriate tyrosine kinase inhibitor to be used as a targeted treatment in order to improve patient outcomes and survival [7,8].
2.1 EFGR testing
EGFR mutations are found in around 10%–12% of Caucasians with adenocarcinoma and are more frequent in never smokers, females and in patients of East Asian ethnicity. EGFR mutation testing is recommended in all patients with advanced nonsquamous cell carcinoma (NSCC) [9]. Molecular EGRF testing is not recommended in patients with an unequivocal diagnosis of squamous cell carcinoma, except in never/former light smokers [10].
EGFR mutations are found in around 10%–12% of Caucasians with adenocarcinoma and are more frequent in never smokers, females and in patients of East Asian ethnicity. EGFR mutation testing is recommended in all patients with advanced nonsquamous cell carcinoma (NSCC) [9]. Molecular EGRF testing is not recommended in patients with an unequivocal diagnosis of squamous cell carcinoma, except in never/former light smokers [10].
Real-time polymerase chain reaction (PCR), Sanger sequencing (paired with tumor enrichment), and next generation sequencing are the most commonly used methods to assess EGFR mutation status [14]. Of the known EGFR tyrosine kinase domain mutations, greater than 90% occur as short in-frame deletions in exon 19 or as point mutations in exon 21, the latter resulting in arginine replacing leucine at codon 858 (L858R). Two less common mutations occur at exons 18 and 21 [15].
The predictive effects of the drug-sensitive EGFR mutations, exon 19 deletion (LREA deletion mutant) and L858R, are well defined. Patients with these mutations have a significantly better response to erlotinib, gefitinib, or afatinib [16]. EGFR T790M is a mutation associated with acquired resistance to EGFR TKI therapy and has been reported in about 60% of patients with disease progression after initial response to erlotinib, gefitinib, or afatinib [17-19].
2.2 ALK translocation
ALK fusion is also encountered, but not exclusively, in never smokers, adenocarcinoma subtype and in younger patients, with a prevalence of around 5% in adenocarcinomas [20,21]. Inversion of the short arm of chromosome 2 leads to the joining of exons 1 to 13 of EML4 and exons 20 to 29 of ALK, resulting in the EML4–ALK chimeric protein, which is known to occur in approximately 4-7% of NSCLC [7].
Several methods for detecting ALK gene rearrangements are available, including fluorescence in situ hybridization (FISH), immunohistochemistry (IHC), reverse transcriptase-polymerase chain reaction (RT-PCR) and next generation sequencing (NGS). There are two different RT-PCR approaches for ALK testing. One technique uses probes for fusion genes (ALK and EML4/KIF5B/ HIP133) [22,23], while the other compares different levels of amplification of small PCR products (50 and 30 portions of ALK transcripts) on the ALK gene (fusion partner independent) [24,25]
Rapid prescreening with IHC to assess for ALK rearrangements can be done; if positive, FISH analysis can confirm ALK positivity [26-28]. Therefore, highly effective ALKTyrosine kinase inhibitors (-TKIs) have been developed, with crizotinib being the first approved agent by both FDA and EMA for the treatment of ALK-rearranged, advanced NSCLC [29].
2.3 ROS1 translocation
ROS1 is an insulin receptor family tyrosine kinase. Its most common translocation aberration is with CD74 and occurs in 1-2% of patients with NSCLC [30]. Aberrant ROS1 kinase activity leads to downstream signaling of the PI3K and MAPK (phosphoinositide 3-kinase and mitogen-activated protein kinase) pathways [31]. NGS can also be used to assess whether ROS1 rearrangements are present, if the platform has been appropriately designed and validated to detect ROS1 rearrangements [32].
Treatment with crizotinib is FDA-approved and recommended for patients with the ROS1 translocation, including those who have received chemotherapy and those who are treatment-naïve [31]. Other TKIs like ceritinib and alectinib are also approved and associated with different response rate and toxicity profiles.
2.4 KRAS mutation
Kirsten rat sarcoma viral oncogene, or v-Ki-ras-2 (KRAS) mutations are present in approximately 30% of pulmonary adenocarcinomas and 5% of pulmonary squamous cell carcinomas [33]. KRAS mutations are also predictive of lack of benefit from EGFR TKI therapy [34-36]. Furthermore, KRAS mutation is mutually exclusive with EGFR mutation and ALK or ROS1 translocation. Once it is identified, there is no further need to test for the other druggable mutations. Nonetheless, there are no available targeted therapy for this mutation.
2.5 BRAF mutation
BRAF (v-Raf murine sarcoma viral oncogene homolog B) is a serine/threonine kinase that is part of the MAP/ERK signaling pathway. BRAF V600E is the most common mutation among the BRAF point mutations; it occurs in 1% to 2% of patients with lung adenocarcinoma [37]. Patients with BRAF V600E mutations are typically current or former smokers in contrast to those with EGFR mutations or ALK rearrangements who are typically non-smokers [38]. Mutations in BRAF do not overlap with EGFR mutations or ALK rearrangements [37,38]. Real-time PCR, Sanger sequencing, and NGS are the most commonly used methods to assess for BRAF mutations.
2.6 HER2
Case series or early-phase clinical trials suggest that patients with tumors harboring HER2 mutations often respond to trastuzumab and chemotherapy, to afatinib (being an EGFR/ HER2 Tyrosine Kinase inhibitor) or to ado-trastuzumab [39]. Other HER2-targeted agents are still under evaluation.
2.7 Other mutations
These are mutations described and reported in NSCLC. Unfortunately, we lack an approved TKI or standard targeted therapy outside a clinical trial when these mutations are present
2.7.1 PIK3CA
Mutations in Phosphatidylinositol-4, 5-Bisphosphate 3-Kinase Catalytic Subunit Alpha are seen in approximately 5% of squamous cell carcinomas of the lung, and numerous agents are under active investigation, with promising response rates at the expense of serious toxicities [40].
2.7.2 DDR2
Discoidin domain receptor tyrosine kinase 2- DDR2 mutations occur in approximately 4% of squamous cell NSCLC. DDR2 is a receptor tyrosine kinase that binds to collagen and promotes cellular proliferation [41].
2.7.3 NTRK1
Neurotrophic receptor tyrosine kinase 1- NTRK1 translocation occurs in <1% of NSCLC and include rearrangements in NTRK1, NTRK2, and NTRK3. NTRK activation leads to downstream signaling through the MAPK and PI3K pathways [42].
2.7.4 MET
Mesenchymal epithelial transition factor receptor -MET is a receptor tyrosine kinase that has a major function in tumor progression when bound to its ligand hepatocyte growth factor (HGF). MET mutations occur in 3% to 4% of NSCLC adenocarcinomas, 2% of squamous cell carcinomas, and 1% to 8% of other subtypes of lung cancer [43,44]. Several agents in preclinical stages are aiming to target the MET exon skipping mutations.
2.7.5 RET
The ’’Rearranged during Transfection’’-RET fusions are present in 1-2% of NSCLC/Adenocarcinoma of patients of both Asian and European descent. Several studies indicate that RET fusion occurs preferentially in young, never‐smoker, and lightsmoker patients [45]. RET fusions are also promising targets for personalized therapy in lung cancer.
2.8 Programmed Death Ligand 1 (PDL1)
The programmed death 1 (PD-1) receptor is an inhibitory T-cell receptor that is engaged by its two known ligands, PD-L1 (also known as B7-H1 or CD274) and PD-L2 (also known as B7- DC or CD273), primarily within the tumor microenvironment. It plays a crucial role in tumor immune escape.
Four different programmed death ligand 1 immunohistochemical assays are currently approved or in development as companion or complementary diagnostics to different immunotherapeutic agents in lung cancer: the Ventana SP142, the Ventana SP263 assay, the Dako 22C3 and the Dako 28-8 assay. Single agent pembrolizumab is approved as first-line therapy for patients with advanced NSCLC with PDL1 expression levels of 50% or more based on a phase 3 randomized trial (KEYNOTE-024) comparing pembrolizumab versus platinum-based chemotherapy [46]. Nivolumab is approved as a subsequent therapy for patients with metastatic NSCLC who have progressed on or after first line chemotherapy based on data from two phase 3 randomized trials (CheckMate-057, CheckMate-017). In the same perspective, based on the Keynote-010, pembrolizumab was approved as a second line treatment of metastatic NSCLC expressing PDL1 in >1% of the tumor cells [47-50]. The regimen consisting of atezolizumab/carboplatin/paclitaxel/bevacizumab is approved as first line therapy for patients with metastatic nonsquamous NSCLC based on a recent phase 3 randomized trial (IMpower150) [51].
Conclusion
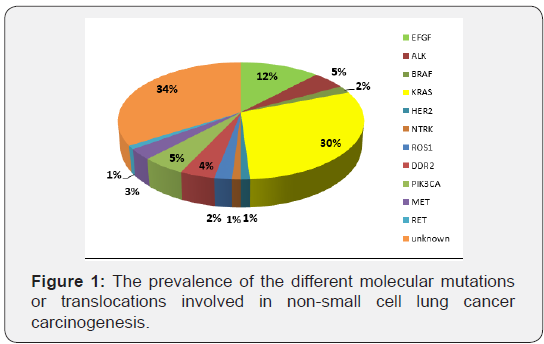
To conclude, the outcome of the patients with metastatic NSCLC has drastically changed with an improved overall survival without affecting the quality of life. Through the knowledge of lung cancer carcinogenesis, the pathologic analysis of the biopsies is no more only dependent on morphological and immunohistochemical features; it is mandatory to thoroughly analyze the molecular profiles and the signaling pathways because many targeted therapies are available. Furthermore, in the era of immunotherapy, new challenges are asked from our pathologist colleagues: the PDL1 expression, the tumor mutational burden and other receptors or protein expressions under investigation. Consequently, new horizons to precision medicine and subsequently to newer therapeutic strategies are currently opened, leading to new toxicity profiles: immunologic, broad spectrum of serious adverse events and mainly an emerging challenging financial toxicity (Figure 1).
To know more about juniper publishers please click on: Juniper Publishers
For more articles in Open
Access Journal of Reviews & Research please click on:
https://juniperpublishers.com/arr/index.php
To know more about Open Access Journals please click on: https://juniperpublishers.com/index.php



Comments
Post a Comment