Preparation, Characterization, and In Vitro Evaluation of Cream & Gel Formulations Containing Lidocaine and Tetracaine- Juniper Publishers
Juniper Publishers- Open Access Journal of Annals of Reviews & Research
Preparation, Characterization, and In Vitro Evaluation of Cream & Gel Formulations Containing Lidocaine and Tetracaine- Juniper Publishers
Abstract
There are multiple different topical anesthetic options available to minimize the pain associated with cosmetic dermatologic procedures. These options are used either alone or in combination and the most used dermal analgesics are lidocaine, tetracaine, prilocaine, or their combinations. EMLA® cream is a local anesthetic that contains lidocaine and prilocaine combination and can be applied to the skin to help suppressing the pain of needle procedures, but it requires occlusion and the length of time for the medication to stay on the skin depends on the type of procedure. It is usually applied at least 1-2 hours before minor skin procedures. The object of this study was to develop topical cream and gel formulations of lidocaine-tetracaine (LT) combination with a suitable consistency to maintain an effective pain alleviation with a faster onset of action which may be an alternative to EMLA® in superficial operations. Two different formulations of LT; a water-in-oil (w/o) emulsion and an emul gel thickened with Carbomer 974 were manufactured. pH levels of emulgel formulations were ranged between 7.2-7.7 at t0, and 8.2-8.9 at t30, respectively. The w/o cream and emulgel formulations showed shear thinning thixotropic behavior at t0 and t30. The morphological properties have been analyzed with a texture analyzer, and properties such as hardness, cohesiveness, and elasticity were calculated. The yield percentage of lidocaine was 65-100%, and tetracaine was 45-100% in emulgels, and 39-79% and 47-67% in creams. In vitro studies showed that LT release was faster in emulgel formulations, and tetracaine release had a longer duration in all formulations. In conclusion, cream and emulgel formulations of LT combination with a suitable consistency, pH level and longer duration of action have been manufactured and evaluated by means of in vitro tests.
Keywords: Local anesthetic; Lidocaine; Tetracaine; EMLA; Cream; Emulgel
Introduction
Drugs that have “membrane-stabilizing activity” have become widespread in clinical practice since Koller discovered the local anesthetic properties of cocaine in 1884. They are now well established in many areas of clinical practice, including nerve blockade, neuropathic pain syndromes, obtunding the pressor response to laryngoscopy, treatment of cardiac arrhythmias, mucosal vasoconstrictors, epilepsy, for treatment of asthma and as a convenient tool for the treatment of patients who may be undergoing superficial dermato surgical procedures [1]. Many topical anesthetic creams have become available over the last few years and they claim to provide a long-lasting anesthetic effect after topical use on the skin. The potential benefits for patch and cream delivery systems over injection include increased safety, the convenience of use, ease of disposal, reduced drug loading, and no tissue dissention [2]. But intact skin presents a significant barrier to available topical anesthetic preparations. This means that many topical anesthetic preparations must be applied at least 45–60 min before the clinical procedure to achieve the desired level of anesthesia [3].
The most commonly used dermal analgesics are lidocaine, tetracaine, prilocaine, or combinations thereof, and the most common topical anesthetic which is regarded as the gold standard is EMLA® cream (Astra Pharmaceuticals, Westborough, MA, USA). EMLA® cream is an emulsion in which the oil phase is a eutectic mixture of 2.5% lidocaine and 2.5% prilocaine (LP). EMLA® cream is a recommended alternative topical anesthetic for radiofrequency ablation if applied under occlusion to the skin at least 1-2 h before the procedure [2]. With EMLA®, analgesia is achieved to a depth of 3 mm after 60 min and a maximum depth of 5 mm after 120 min of EMLA® exposure. EMLA® has been shown to provide effective anesthesia for several different procedures but requires occlusion and does not provide adequate anesthesia at peripheral skin margins [4]. Food and Drug Administration (FDA) has approved a stable compounded mixture of 7% lidocaine and 7% tetracaine (LT) cream in 2006. The lidocaine and tetracaine (LT) patch is a drug delivery patch that uses a disposable, oxygen-activated heating element to emit controlled heat to enhance drug delivery of a thin, uniform layer (ZARS, Inc., Salt Lake City, UT, USA). The heating element generates a controlled level of mild heating (generating a skin temperature of 39 to 41 °C) during the application period [5]. Topical anesthesia with a waiting time of 30 minutes would be more viable and convenient to use in dermatology procedures compared to the one requiring a downtime of at least 1 hour [2].
One of the benefits of topical local anesthetics is effective anesthesia for certain procedures with little to no systemic exposure to the agent. However, as seen from the approved formulations, topical anesthetics are often limited by the need for occlusive dressings in order to enhance their epidermal penetration. In addition, inadequate or non-uniform placement of anesthesia over the skin area, messy application, and need for a lengthy application period are other limitations of topical anesthetics. Ideally, topical anesthetics must be able to penetrate the relatively impermeable barrier of the stratum corneum and have minimal systemic absorption [4]. The rationale for the LT combination is due to the pharmacokinetics of the two components. The anesthesia produced by lidocaine is faster, more intense, longer lasting, and more extensive than that produced by an equal concentration of procaine. Tetracaine, a long-acting amino-ester, is more lipophilic than lidocaine, concentrating in the stratum corneum of the epidermis, where it slowly diffuses. Its duration is thus prolonged and systemic uptake is limited [6].
An attempt has been made in the present work to develop suitable topical formulations of LT combination which may be an alternative to EMLA®, that has a faster onset of action and longer duration of anesthesia, where occlusion is not needed before the procedure and can be used in not only small dermosurgical operations but superficial operations that last longer in either emulgel or cream form. Physiochemical and In Vitro studies were performed for formulation characterization.
Materials and Methods
Materials
Lidocaine (base) was given as a gift by Apex Healthcare Ltd., tetracaine (base) was given as gifts by Jinan Chenghui-Shuangda Chemical Co. Ltd., Sepineo P600 was given as a gifts by SEPPIC, cetyl alcohol, stearyl alcohol, white petrolatum, propylene glycol, methyl parabene, and NaOH were provided from Aklar Chemical, sodium lauryl sulphate (SLS) was provided from Emir Chemical, castor oil was provided from Loba Chemie, and Carbomer 974 P was provided from Lubrizol. Deionized water was obtained from a Millipore water supplier. Calculations were performed on Microsoft Excel 2010 and statistical tests were executed on Minitab® 17.1.0.
Methods
Formulation Preparation
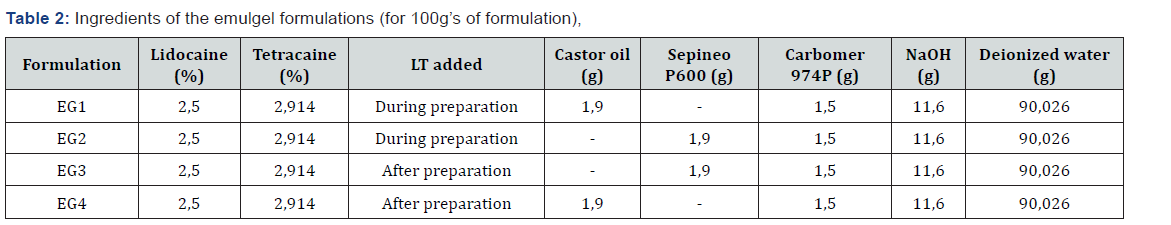
Two different methods were used in the preparation of cream and emulgel [7] formulations. The difference between the two methods was adding the active substances whilst or after the base preparation. The purpose was to see if a eutectic mixture occurs, and if so, its effect on the preparation process and homogeneity of formulations. The cream formulations were coded with “C”, and emulgel formulations with “EG” (Table 1&2).
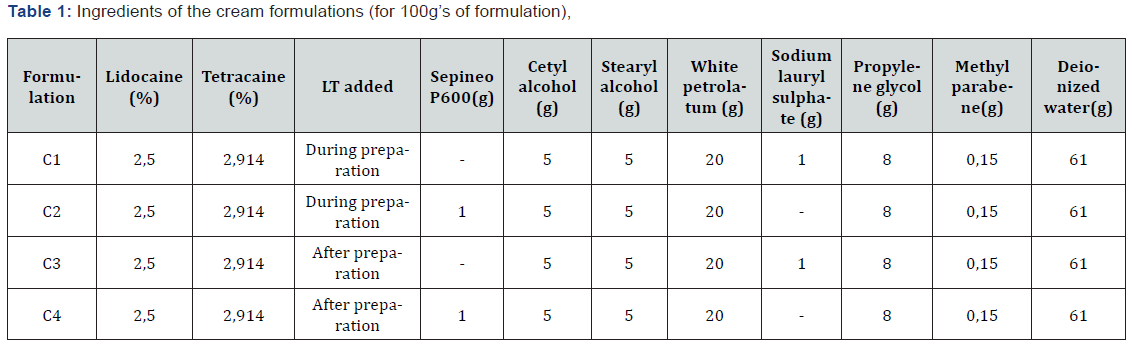
a) Cream preparation method 1: The oil phase was prepared by melting the cetyl and stearyl alcohols and forming cetostearyl alcohol, adding white petrolatum and warming the mixture to 70 °C. The aqueous phase was prepared by dissolving SLS or Sepineo P600 that was used as the emulsifying agent in the formulations, propylene glycole and methyl parabene in deionized water. The aqueous phase was warmed to 70 °C until all ingredients were dissolved. The aqueous phase was slowly added to the oil phase with moderate agitation and was kept stirred until the temperature dropped to 40 °C. The emulsion was cooled to room temperature to form a semisolid cream base. The mixture was stirred until the formulation became uniform. Lidocaine and tetracaine were added to the formulation.
b) Cream preparation method 2: The oil phase was prepared by melting the cetyl and stearyl alcohols and forming cetostearyl alcohol, adding white petrolatum and warming the mixture to 70 °C. Lidocaine and tetracaine were added to the oil phase. The aqueous phase was prepared by dissolving SLS or Sepineo P600 that was used as the emulsifying agent in the formulations, propylene glycole and methyl parabene in deionized water. The aqueous phase was warmed to 70 °C until all ingredients were dissolved. The aqueous phase was slowly added to the oil phase with moderate agitation and was kept stirred until the temperature dropped to 40 °C. The emulsion was cooled to room temperature to form a semisolid cream base. The mixture was stirred until the formulation became uniform.
c) Emulgel preparation method 1: The deionized water was heated and Carbomer 974 P was dissolved in 70% of the water. The pH level was adjusted to a range of 9-9,5 with 2N NaOH solution. Carbomer gel was heated to 70 °C. Castor oil or Sepineo P600 that were used as the surfactant was heated to 70 °C, slowly added to the gel and was stirred until the mixture was uniform. Remaining deionized water was heated up to 60 °C and was added to the mixture and stirred until the formulation cooled down and became uniform.
d) Emulgel preparation method 2: The deionized water was heated and Carbomer 974 P was dissolved in 70% of the water. The pH level was adjusted to a range of 9-9,5 with 2N NaOH solution. Carbomer gel was heated to 70 °C. Castor oil or Sepineo P600 that were used as the surfactant was heated to 70 °C, slowly added to the gel and was stirred until the mixture was uniform. Remaining deionized water was heated up to 60 °C and was added to the mixture and stirred until the formulation cooled down and became uniform. Lidocaine and tetracaine were added to the formulation.
Preparation of standard curve and analytical method
Standard calibration curves were constructed for lidocaine and tetracaine in order to obtain the linear equations which were further used to calculate the yield percentage and In Vitro release of LT. For this purpose, stock solutions of lidocaine and tetracaine were prepared by dissolving 5mg of lidocaine or tetracaine in 1 mL of ethanol and diluting to 50 mL of phosphate buffer saline (pH 7.4) (PBS). Stock solutions were further diluted with PBS for final concentrations and were measured spectrophotometrically (Thermo Scientific Evolution 201 UV Visible Spectrophotometer) using a wavelength of 205nm for lidocaine and 311nm for tetracaine.
Characterization of LT emulgel and creams
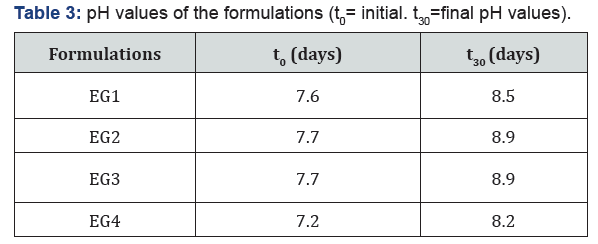
pH measurements: The pH of the formulations (Eutech Instruments pH 700) were measured at room temperature (25 °C). Samples were prepared by diluting 0,2g of emulgels in 20 mL of deionized water [8-10].
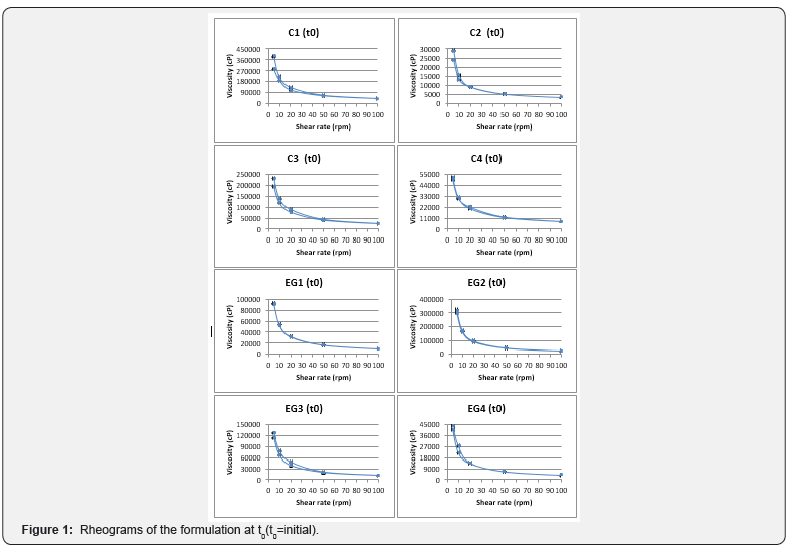
a. Rheological measurements: The viscosities of all formulations were measured with Brookfield Viscometer (Model DV-II, Brookfield Engineering Laboratories, Inc., Stoughton, MA) equipped with spindle number 96. All the emulgel and cream formulations were subjected to a continuous variation of shear rate from 5rpm to 100rpm and 100rpm to 5rpm, and the viscosity was measured (n=3). The rheological behavior of the formulations was evaluated by constructing rheograms where the viscosity (cP: centipoise) versus shear rate (rpm) was plotted and their rheological properties were analyzed.
Quantification of lidocaine and tetracaine in creams
For the extraction of LT from the creams, 0.2g of each cream was placed in a test tube and was dissolved in 1mL of 0.1 N NaOH, followed by addition of 2mL methylene chloride. All mixtures were vortexed for 1 min and centrifuged for 5 min at 2000rpm. The extraction was repeated three times for each sample, and the solvent layer was collected and combined. After evaporation of methylene chloride, the residue was reconstituted with 1mL of phosphate buffer saline (pH=7,4) and analyzed spectrophotometrically [11].
Texture profile analysis
Texture profile analysis (TPA) was performed using a TAXT2 Texture Analyser (Stable Micro Systems, Surrey, U.K.) in TPA mode. Formulations were transferred into 25 mL beakers. A cylindrical analytical probe (0,5inch diameter) moved down slowly and penetrated each sample at a defined rate (1mm/s) and to a defined depth (6mm). Three replicate analyses of each sample were performed at room temperature (25 °C). From the resulting force – time plots; hardness, elasticity, and cohesiveness were derived [12].
Stability studies
The emulgel and cream formulations were put in plastic and glass jars (40g) and subjected to stability studies at 25 °C, 60% RH for a period of 30 days. Samples were evaluated for physical appearance, rheological properties, texture profile and In Vitro LT release.
In Vitro LT release studies
Each formulation was weighed and placed in a Franz diffusion cell, and a semipermeable cellulose membrane with a molecular weight cutoff point of 14,000 Da which previously was immersed in receptor phase overnight was placed over the mouth of the cell. The cells were immersed so the membrane was touching the receptor phase. The receptor phase was a volume of 200mL Phosphate Buffer Saline (pH=7,4) which was maintained at a constant temperature of 37° C ± 1°C in a water bath, and stirred by a magnetic stirrer at 600rpm. Intervals of 15, 30, 45, 60, 90, 120, 180, 240, 360, 480, 600, 720, 1440 and 1560 min were chosen as sampling times, and 2mL aliquots were taken and replaced with an equal volume of receptor phase. Samples were analyzed for lidocaine and tetracaine content spectrophotometrically at 205 and 311nm respectively. The mechanism of drug release from the formulations was studied from the drug release data using the modeling equations as follows:
Q = K1t (1)
Log Qo= K2t/2.303 (2)
Q = K3t1/2 (3)
where Q is the percentage drug released, Qo is the percentage of drug unreleased at time t; K1, K2, and K3 are the release rate constants for zero order kinetics (1), first-order kinetics (2) and Higuchi’s square root of time diffusion equation (3) [13].
Results and Discussion
Characterization of LT emulgel and creams
All formulations were white in appearance, homogeneous and odorless after inspection. As shown in the Table 3, all the formulations are within the physiologic pH range accepted for dermal preparations [14] which would indicate a slightly basic environment, but not significant enough to compare with EMLA® cream, which is highly alkaline (pH 9.0) [15]. Rheologic measurements showed that all the systems showed thixotropic shear-thinning behavior as shown in Figure 1.
Quantification analysis
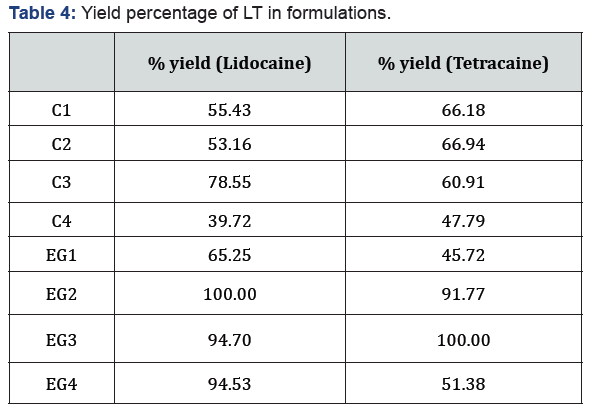
Yield percentage of LT according to quantitative analysis are shown in Table 4. It has been observed that the recovery yield percentage of LT combination was higher in emulgel formulations than cream formulations.
Texture profile analysis
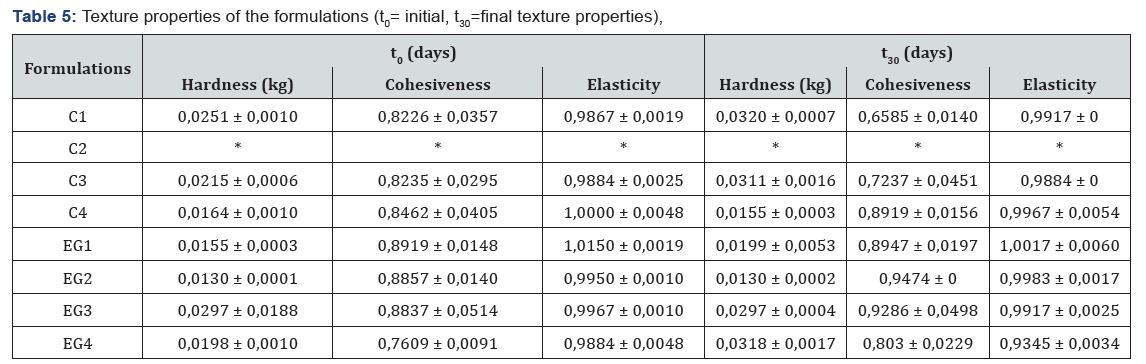
Texture profile analysis defines the mechanical parameters in terms of hardness, cohesiveness, and elasticity. The hardness expresses the necessary force to deform semi-solid formulations or the applicability of the gel to the desired site. Formulations should have low hardness value to be administered to the skin easily. The cohesion introduces the measure of the reconstruction of the semi-solid formulation after application. The high value of cohesiveness means full structural recovery following application. Elasticity is defined as the direction of reconstruction of the gel after its deformation by compression in the means of time [16]. Texture profile parameters were presented in Table 5.
In Vitro drug release studies
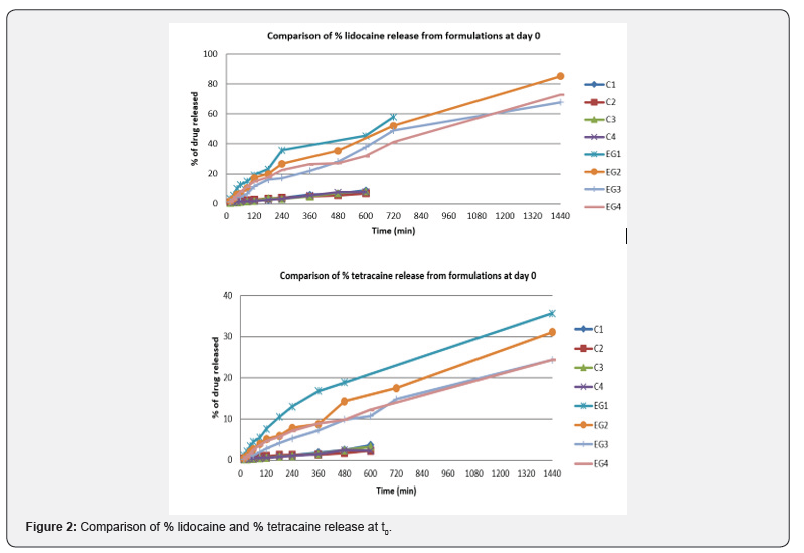
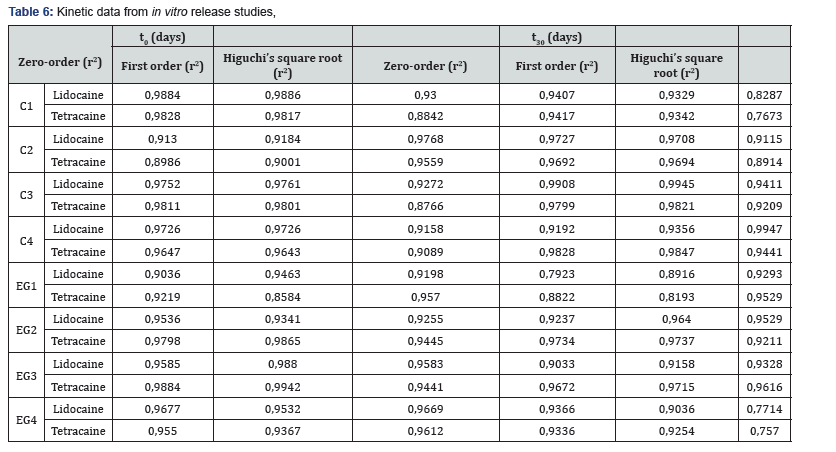
As seen in Figure 2, emulgel formulations released the LT combination more rapidly than the cream formulations. All preparations are listed according to the release rate of active substance: EG1, EG2, EG4, EG3, C2, C1, C4, and C3, respectively. The formulations that LT was added during the preparation in the gel formulations and the formulations which contain castor oil as surfactant have been found to provide faster release of LT than those containing Sepineo P600. Formulations which LT was added during preparation, and contain Sepineo P600 as surfactant appeared to provide faster lidocaine release, and tetracaine release had a longer duration. The regression data obtained from the kinetic indicated that the data fitted better to first order kinetics (e.g. C3, C4, EG2 and EG3) and which gave higher linear regression coefficient for the formulations. It can be said that release rate from the formulations is dependent on the concentration of drugs Table 6.
Stability studies
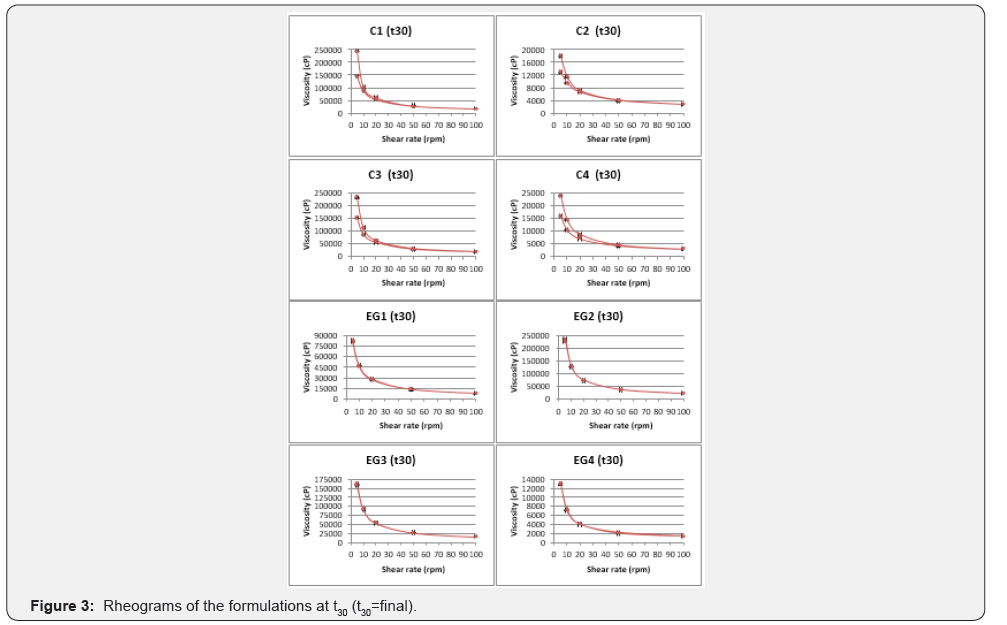
Stability studies were carried out to detect any changes in pH, rheologic properties, and LT release profile through 30 days of storage at 25 °C and 60% relative humidity. All preparations were physically stable retaining homogeneity with no phase separation after 30 days. As shown in Table 3, pH levels of the formulations increased after 30 days (Table 3). Therefore, it is thought that gel formulations should be used immediately after preparation or should be stored at temperatures below 25 °C. After the stability studies, the viscosities of all formulations except EG3 decreased (Figure 3). However, all formulations continued to exhibit thixotropic, shear thinning behavior in terms of rheological properties. After the stability studies, hardness increased in all formulations except C4, cohesiveness increased in all formulations except C1 and C3 (Table 5). The difference between elasticity values has been found not to be statistically significant (p > 0,05). After the stability studies, LT release profile of the formulations highest to lowest was EG2, EG1, EG3, EG4, C2, C4, C1, and C3 respectively (Figure 4). The formulations in which LT was added during the preparation showed more rapid release than the other formulations. It is thought that the LT added after which was not solubilized in the formulation solubilized with the temperature provided with In Vitro and stability studies [17], therefore the release rate was reduced. The formulations that contained Sepineo P600 as a surfactant released the LT combination more rapidly than the other formulations. It is thought that the change in release profiles may be due to degradation of castor oil (organic surfactant).

Conclusion
The objective of this study was to develop suitable emulgel and cream formulations of LT combination which might be an alternative to EMLA® and to develop formulations which have a faster onset of action and longer duration for anesthesia. Manufactured formulations had suitable pH and viscosity values for topical application, and released lidocaine rapidly and tetracaine in a controlled manner. Emulgel formulations exhibited more rapid release of the LT combination than the cream formulations, therefore making them more suitable for dermo surgical operations. Our further studies will focus on comparative in vivo studies and clinical investigations. If their clinical benefits are confirmed, because tetracaine’s anesthetic and long-term effects are more potent than prilocaine, these formulations can provide a better alternative to EMLA® regarding effectiveness and convenience in clinical practice.
To know more about Juniper Publishers please click on: https://juniperpublishers.com/aboutus.php
For more articles in Open
Access Journal of Reviews & Research please click on:
https://juniperpublishers.com/arr/index.php


Comments
Post a Comment