Ecto and Endo Parasites of Domestic Birds in Owan West, East and Akoko-Edo in Edo State of Nigeria- Juniper Publishers
Juniper Publishers- Open Access Journal of Annals of Reviews & Research
Ecto and Endo Parasites of Domestic Birds in Owan West, East and Akoko-Edo in Edo State of Nigeria- Juniper Publishers
Abstract
Twenty-three (23) live domestic birds comprising of 13 chickens (Gallus gallus domestica), 6 ducks (Anas sparsa), and 4 pigeons (Columbia livie) distributed across 3 Local Government Council Areas (LGAs) in Owan West, East and Akoko-Edo of Edo State, Nigeria, was screened for ecto- and endo-parasites. In addition, two hundred (200) faecal samples from 200 domestic birds distributed across the 3 LGAs were investigated for endoparasites. From the 23 live birds, nine species of ectoparasite (Mallophaga-lice), were recovered, of which Menopon gallinae recorded the highest prevalence (23.33%), while Coclutogaster heterographus recorded the least (3.33%). 162 of the 223 birds (23 live birds + 200 faecal samples) investigated were positive for endoparasites recording an overall prevalence of 72.65%. From these hosts, 25 species of endo-helminths comprising of 9 species of cestode; 13 species of nematode; 3 species of trematode and some protozoan sporocysts were recovered. Raillietina tetragona recorded the highest overall prevalence (20.40%), while Amidostomum anseris recorded the least with a prevalence of 0.20%. All 13 live chickens (100%) and 2 pigeons (50%) were observed to be infected with endoparasites. They included: 8 species of cestode; 3 species of nematode and a trematode species. R. tetragona recorded the highest overall prevalence (24.69%) while A. anseris recorded the least with a prevalence of 0.94%. A total of 147 (73.50%) of the 200 faecal samples investigated recorded various species of endoparasites. They included: 12 species of nematode (63.39%); 6 species of cestode (29.46%); 2 species of trematode (6.25%) and some unsporulated protozoan sporocysts (0.89%). Heterakis gallinarum recorded the highest prevalence (21.88%) while A. anseris recorded the least (0.45%). These observations showed that the recorded ecto-and endo-parasites are major consequences of the bird’s migratory and feeding habits.
Keywords: Ecto and endo parasites; Domestic birds; Feeding habits and Parasitic infections
Introduction
The rearing of domestic birds, especially chicken has been a common preoccupation of inhabitants of both the rural and peri-urban centres in Nigeria, who use their eggs and meat mainly as sources of animal protein and farm manure [1-3]. Some of these birds are reared in free-range where they are allowed to move freely within the neighbourhood scavenging for food and water for themselves and their young and retire to their pens at night to sleep. Furthermore, the pens of these birds where available are not properly managed as they are usually overcrowded and in very poor and unhygienic state with no form of veterinary care [4]. These practises have captured the attention of researchers around the world, as these predispose them to disease infection and transmission that are of health and economic importance to the birds and farmers respectively. Disease infection caused by poor hygiene and absence of veterinary care among domestic birds has been implicated as an important cause of low productivity in poultry as it accounts for about ¾ of a billion deaths in Africa annually [5].
These free-range domestic birds scavenge on a variety of food materials and / or lower living organisms such as grains, general wastes, fruits, faeces, worms, various insects, snails, etc., found on plants, and in/on contaminated soils [4]. Some of these food materials are contaminated with various pathogens, while some of the bird preys serve as intermediate hosts harbouring infective stages of parasites, thus predisposing them to various ecto and endo parasitic infections [6-8]. The skin and feathers of domestic birds have been known to harbour a bewildering number of ectoparasite species [9], some of which are able to penetrate the skin and air sacs. These ectoparasites are lice (Mallophaga), fleas (Siphonapera), ticks and mites (Acarina) [7]. Many species such as the lice consumes dead cells of the skin or the epithelia debris while others like fleas, ticks and mites feed on blood.
Due to the feeding habit of these ectoparasites or arthropods, most of them cause great irritations to domestic birds as well as act as vectors of various parasites [9]. These result in anaemia, reduced reproduction of young, weakness, listlessness and emaciation, subsequently leading to poor harvest, owing to premature deaths of the domestic birds while countless others suffer chronic and debilitating effects [9,10] further remarked that the invasion of these parasitic arthropods constitutes the most important cause of losses in poultry industries particularly in the tropics. Domestic birds in addition to their ectoparasites harbour many endoparasites, such as bacteria, viruses, protozoans and helminths especially. Gastrointestinal parasites which invade the host possess morphological and physiological features such as small thread-like cylindrical body, hooks and hard body cuticle which enhance their adaptation to long living and existence in their hosts. Heavy gastrointestinal helminthiasis is characterised by emaciation, mucoid diarrhoea, loss of appetite, anaemia, weakness, paralysis and death. Also associated with parasitic infections are catarrhal inflammation, maceration and thickening of gastrointestinal tracts [11].
Infection of domestic birds with multiple helminth species is common in poorly managed poultries where birds are reared in large numbers without movement restrictions which further complicates the spread of these parasites resulting to poor health and development. These parasites constitute a major factor limiting productivity of the poultry industry by affecting the growth rate of the host resulting in malfunctioning of organs and eventually death [7]. This paper reports on the occurrence of ectoand endo-parasites of three species of domestic birds; domestic chickens (Gallus gallus domestica), pigeons (Columba livie), ducks (Anas sparsa) in Owan West, Owan East and Akoko-Edo Local Government Areas (LGAs) of Edo State, Nigeria to determine the prevalence of infection in each of the three hosts and in the three LGAs. Information so derived could help assess the impact of parasitic infections in/on domestic birds in all three LGAs.
Material and Methods
Study location
The study was conducted in (15) fifteen communities in three (3) LGAs of Edo State, Nigeria namely; Owan West (Avbiosi, Eme- Ora, Sabongida-Ora, Ukhonmora and Uzebba), Owan East (Afuze, Ojavun, Otuo, Ihiebve, Uokhai) and Akoko-Edo (Akuku, Enwan, Igarra, Ikpeshi and Sasaro).
Sample size
223 samples (23 live domestic birds and 200 faecal samples) from 223 domestic birds were examined in the 3 LGAs. The 23 live domestic birds which comprised of 13 chickens, 6 ducks and 4 pigeons were investigated for ecto and endo parasites while the 200 faecal samples were examined for endoparasites as follows: Owan West: 50 (chicken 45, duck 2, pigeon 3), Owan East: 50 (all chickens), and Akoko-Edo: 100 (chicken 98, pigeon 2). The age of the live domestic birds was determined by examining the iris colour and unmolted feathers as described by [12]. After purchasing, the domestic birds were transported in cages to the Parasitology laboratory where they were examined, humanely killed by cervical dislocation and examined for ecto and endo parasites. The faecal samples were collected early in the morning from the pens of free-range domestic birds from the various households in the 15 communities, using a spatula and placed individually into sterile plastic containers and labelled. These samples were analysed using the direct method of Scholten et al. [13] and that of spontaneous sedimentation by Greve et al. [14].
Investigation of Parasites
Ectoparasite investigation
The ectoparasites were collected as described by Soulsby [7].
Briefly after killing the birds separately by decapitation, each was
immediately placed in separate polythene bags and the parasites
collected after losing attachment from the skin and feathers
of the birds. The ectoparasites were sorted and preserved for
identification in 70% alcohol.
Post-Mortem and Endo-Helminth Investigation
The post-mortem examination of 23 live birds was done
according to Fowler [15]. After decapitation, the abdominal and
thoracic cavities were opened, followed by systemic autopsy
examination which include, the oesophagus through to the
gizzard, the small intestine, duodenum, (jejunum and ileum), the
caeca, and the ileocecal junction to the cloaca. Each section was
opened longitudinally. The intestinal scrapping and floatation
methods were used to collect the parasites [7,16]. Finally, the
contents were examined under stereomicroscope and all endohelminths
were counted before being fixed in 70% ethanol for
further identification using keys from Soulsby [7].
Identification of Parasites
Ectoparasite identification
Recovered ectoparasites were identified with the aid of tables and keys by Soulsby [7].
Endo-Helminth identification
Recovered endo-helminths were cleared in lactophenol and
examined for taxonomic morphological features with the aid of a
light microscope under the 10x objective lens. All parasites were
identified using the helminthological keys in Soulsby [7].
Data Analysis
The prevalence of parasites was calculated using the formula
(P=d/n). ‘P’ represents prevalence, ’d’ represents the number of
individuals having an infection at a point in time and ‘n’ is the
number of individuals in the population at risk. This was calculated
according to Thrustfied [17].
RESULTS
Prevalence of Ectoparasites in Live Birds
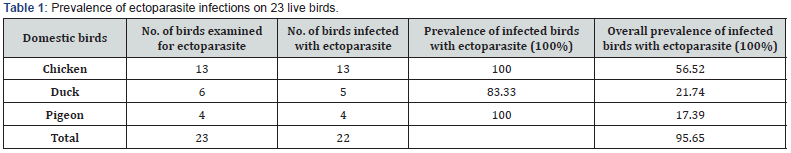
23 live domestic birds comprising of 13 chickens (Gallus domestica), 6 ducks (Anas sparsa) and 4 pigeons (Columba livie) were investigated for the presence of ectoparasites (Table 1) as well as for endoparasites. 22 of the 23 domestic birds investigated harbored at least one species of ectoparasite recording an overall prevalence of 95.65% (Table 1). Results in Table 1 show that all 13 examined chickens as well as all 4 pigeons were found to be infected with ectoparasites, hence recording a prevalence of 100% each, while 5 (83.33%) of the 6 live ducks were also infected (Table 1).
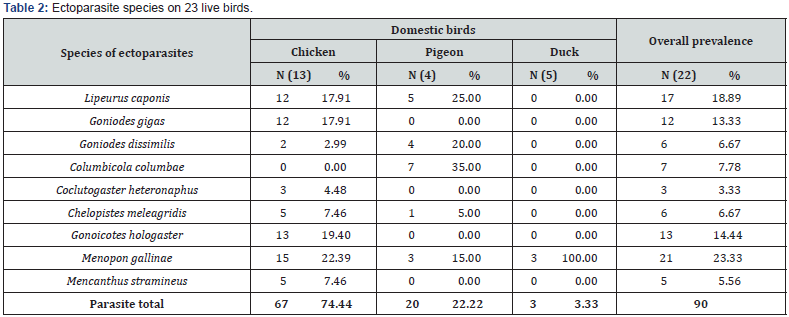
Table 2 shows that nine species of lice were recovered from the body of the birds. Menopon gallinae recorded the highest overall prevalence (23.33%) in all three species of domestic birds, while Coclutogaster heterographus recorded the least (3.33%). Also, Menopon gallinae was the most prevalent ectoparasite recovered from chickens and ducks with prevalence of 22.39% and 100% respectively, while Columbicola columbae recorded the highest in pigeon with a prevalence of 35.00%. On the other hand, Columbicola columbae recorded no prevalence on chickens; Goniodes gigas, Coclutogaster heterographus, Goniocotes hologaster and Menacanthus stramineus recorded no prevalence on pigeons; while Menopon gallinae was the only species of ectoparasite observed on ducks.
Prevalence of Endoparasites in Live Birds
Of the 23 live domestic birds investigated, 15 (65.22) were found to harbour at least one species of endoparasite. All the 13 (100%) live chickens examined were found to be infected with endoparasites, 2 (50%) of the 4 examined pigeons comprising of 8.70% of the total (23) live birds examined was also infected, while none of the ducks examined was infected (Table 3).
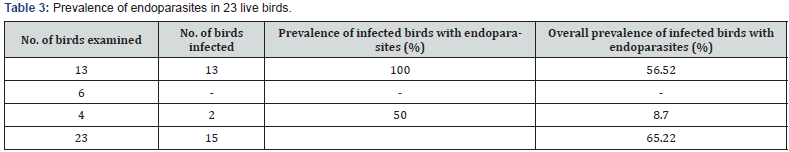
Prevalence of Endoparasites In Live Birds and Faecal Samples
Of a total of 223 domestic birds investigated for endo-helminths (200 faecal samples and 23 live domestic birds), 162 were observed to be infected with various species of endoparasites, recording an overall prevalence of 72.65%. From these hosts, 25 species of endo-helminths and some unsporulated protozoan oocysts was recovered and they included; 9 species of cestode which were most prevalent (59.60%), 13 species of nematode (36.64%) and 3 species of trematode (4.36%), while the unsporulated protozoan oocysts recorded a prevalence of 0.40%. Raillietina tetragona recorded the highest overall prevalence of 20.40% while Amidostomum anseris recorded the least with a prevalence of 0.20% (Table 4).
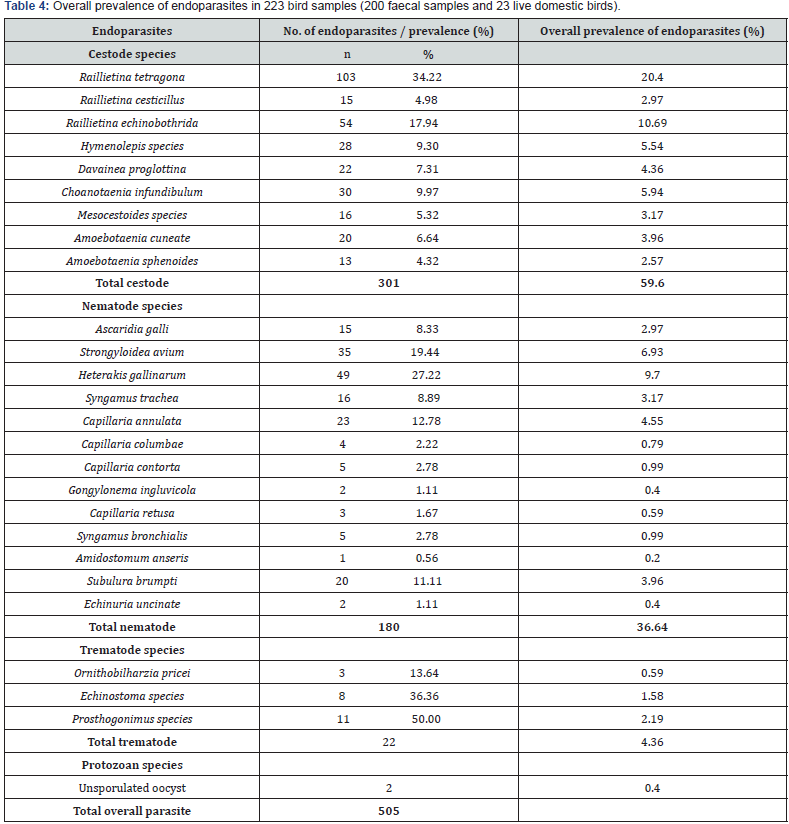
Endoparasite Infections in the 23 Live Domestic Birds
In the 23 live domestic birds examined, eight of the nine species of cestode recorded from the 223 samples (23 live birds and 200 faecal samples) was recorded, 3 of the 13 species of nematode was demonstrated while Echinostoma species was the only trematode species that was observed. R. tetragona recorded the highest overall prevalence (24.69%) in all three species of live birds examined while A. galli (0.94%) recorded the least. Also, R. tetragona recorded the highest prevalence (30.38%) in chickens while C. contorta recorded the least with no prevalence. R. echinobothrida, A. cuneata and C. contorta were the only endoparasite recorded in pigeons with prevalence of 85.00%, 5.00% and 10.00% respectively. None of the 6 live ducks examined was infected with endoparasite (Table 5).
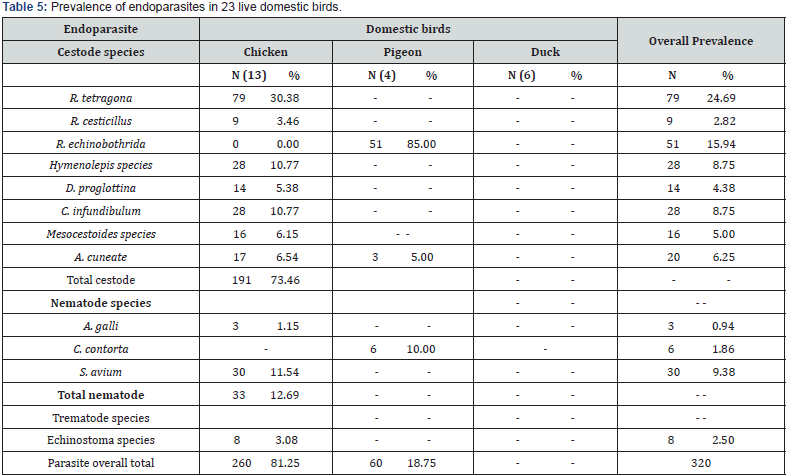
Endoparasites in 200 Faecal Samples From 200 Live Birds
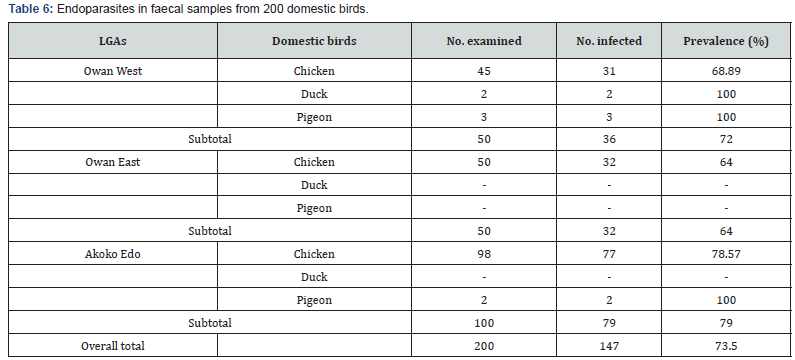
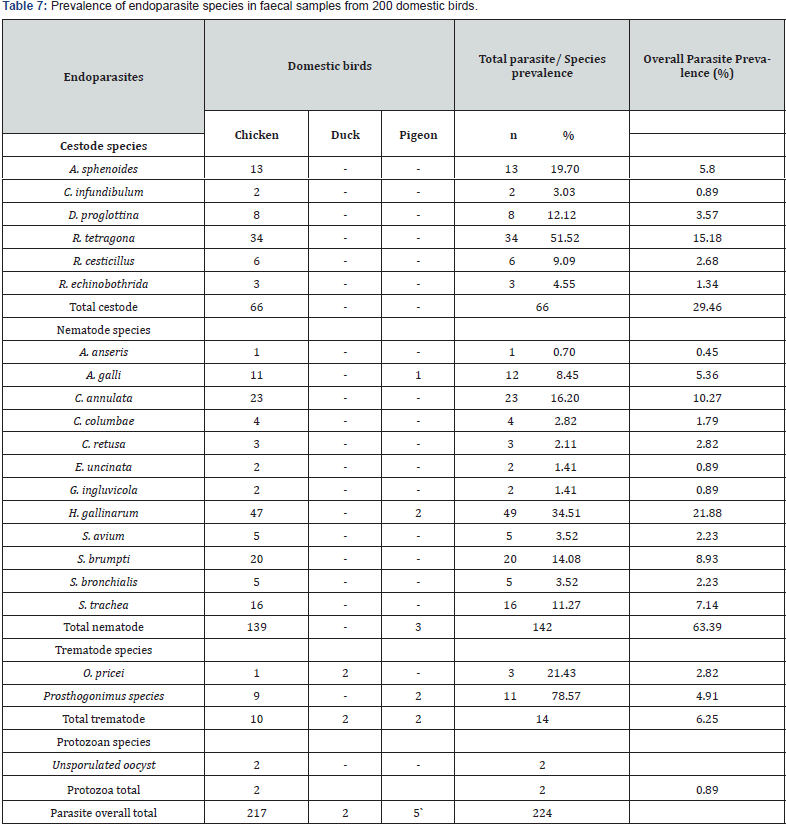
Of the 200 faecal samples examined, 147 (73.50%) were observed to be infected with various species of endoparasites. Among the three LGAs, birds from Akoko Edo recorded the highest parasite prevalence (79.00%), followed by Owan West (72.00%) and Owan East (64.00%) (Table 6). Twenty-one (21) species of endo-helminths and 2 unsporulated protozoan oocysts were recorded. Endo-helminths comprised of 6 species of cestode, 12 species of nematode and 2 species of trematode. Nematodes were the most prevalent (63.39%), followed by cestodes (29.46%), then the trematodes (6.25%) and the least was the protozoan oocysts (0.89%). H. gallinarum recorded the highest overall prevalence of 21.88% while A. anseris recorded the least with an overall prevalence of 0.45% (Table 7).
Discussion
Ectoparasite prevalence in Domestic Birds
Findings in this report show a high prevalence of ectoparasites in Owan West, Owan East and Akoko-Edo LGAs in Edo state of Nigeria. This could be attributed to the fact that these birds are reared in free-range where they are left at their mercy to scavenge daily for food on sand, dust particles, dump-sites, etc.; a common abode for most lice. The high prevalence of lice on these birds is further complicated with the fact that they are not given any form of body treatment. More so, the role of mother hen in providing shelter for their young with the aid of her feathers and the over crowdedness of these birds in pens also ensured the spread of these lice from bird to bird. Lice-induced itching can cause skin damage or injuries to domestic birds which in severe cases can negatively affect egg production as well as quality and quantity of poultry yield as birds spend more time in preening rather than in other productive activities [18].This finding here corroborates with previous reports by Edosomwan et al. [19]; Trees and Beesly [20], that lice such as M. gallinae, M. stramineus and L. caponis are predominant on domestic birds especially chickens. Also, findings herein corroborate with that of Msoffe et al (2009), who reported M. gallinae and M. stramineus on pigeons in Morogoro Municipality, Tanzania, though with low prevalence of 0.50% each, but reported a high prevalence of Pseudolynchia carariensis (61.50%) which is not found in this study. Furthermore, the observation of C. columbae as the most prevalent ectoparasite in pigeons herein concurs with report by Harlin [21]. This study also recorded C. columbae (the slender pigeon louse) and M. gallinae (the fluff louse of chicken and guinea-fowl) both belonging to the order Mallophaga (biting lice), suborder Ischnocera, family pediculidae, which had also been reported previously by Soulsby [7].
Endoparasite Prevalence in Domestic Birds
The findings revealed a high prevalence of endoparasites, particularly helminths in domestic birds of the study areas. This could be due to reason that the birds are reared in free-range (that is no form of food or water or drugs) are provided for them. Therefore, the birds are left to fend for themselves and their young. Thus, they undergo various scavenging activities on faeces, debris and waste / refuse dumps where they encounter and feed on a wide range of diets that predispose them to parasitic infections [6], with many of the foods from contaminated soils carrying infective intermediate stages of endo-helminth parasites such as eggs or larva that are infective to domestic birds of free-ranging [2]. Also, the high prevalence of endo-helminths in the domestic birds could further be complicated by continuous ingestion of infected droppings or infected intermediate host of organisms such as beetles, cockroaches, earthworm, flies, and grasshoppers that are readily available to them in poorly managed stocks [22,23]. Their digging activities in search for food for themselves and their young and lying on open soil for warmth also expose them to endo-helminth infections.
The high prevalence of H. gallinarum and other nematodes in domestic birds could also be attributed to their fairly developed digestive system which gives them greater chances of establishing a host-parasite relationship [24]. The presence of certain conditions especially moisture appears to favour high burdens of parasitic helminths in domestic birds particularly those with a direct life cycle [25,26]. The prevalence of R. tetragona (20.40%), R. echinobothrida (10.69%), H. gallinarum (9.70%), R. cesticillus (2.97%), C. infundibulum (5.94%), A. galli (2.97%), S. trachea (3.17%), G. inglovicola (0.40) and other helminths in this study corroborates with the report of [24] on the gastrointestinal parasites of domestic chickens in Zaria, Nigeria. The presence of coccidians in this study is also in line with those of Marieto- Goncalves [27], who reported coccidians as the most common parasite in his analysis of birds. It also agrees with the data given by Freitas et al. [28] who observed a high prevalence of coccidian oocysts as well as endo-helminths, such as Heterakis species, eggs of Ascaridia and capillaria species in the faecal samples of wild birds in Brazil. Also, eggs of Ascaridia species, Strongiloidea species and Capillaria species have been reported by Cunha et al. [29] in a study of wild birds in Brazil. Furthermore, also reported gastrointestinal helminths in free range ducks in Morogoro, Tanzania. Three species of cestode; R. tetragona, R. cesticillus, R. echinobothrida and a nematode (A. Galli) reported herein, although, mostly in chickens have been reported elsewhere in a study of domestic chickens and ducks from Gombe state, Nigeria [30] and in chickens from Kaduna state, Nigeria [31]. A similar investigation by Msoffe et al. [32] also recorded the same parasites in nestlings and adult pigeons in Morogoro, Tanzania. Also, according to the same author, R. echinobothrida was shown to be an important cestode parasite of pigeons. Although, it is generally considered to be a relatively harmless parasite, it will be interesting to study the reason(s) why pigeons appear to be more susceptible to the above parasite compared to ducks and other birds. The report herein is also in line with Yusufu et al. [33] who reported R. tetragona (78.8%), Hymenolepis species (12.1%), A. cuneata (6.1%) in Quela birds in Borno state, Nigeria, in a study of the relationship between their feeding habits and gastrointestinal parasites.
The low prevalence of endoparasites in ducks in this study could be attributed to their feeding habits [34], as they have very limited access to free water in the study area, which is a semi-arid region, thereby limiting their exposure to snail intermediate hosts which are the carriers of larval trematodes [30,35] Findings herein that chickens could be more susceptible to parasitic infections when compared to pigeons and ducks concur with previous reports [19,30]. However, further investigations are needed to ascertain this.
Conclusion
In conclusion, the high prevalence of Ecto and endoparasites observed in domestic birds in this study reinforces the data reported in Goulart [36], who stated that Galliformes, as well as other ground-level feeders are more susceptible to constant re-infection by parasites. Thus, proper poultry management and hygiene needs to be put in place to ensure good health and in turn high productivity of domestic birds and other poultry, whose products could serve as alternative sources of income and food protein to local inhabitants / farmers if properly harnessed [37,38].
To know more about Juniper Publishers please click on: https://juniperpublishers.com/aboutus.php
For more articles in Open
Access Journal of Reviews & Research please click on:
https://juniperpublishers.com/arr/index.php



Comments
Post a Comment